
Pablo Lara-Gonzalez, PhD
Dr. Pablo Lara-Gonzalez is originally from Chile, where he completed his Bachelor’s degree in Biochemistry at the Universidad de Concepcion. He then obtained his PhD at the University of Manchester in the United Kingdom investigating the molecular mechanisms of mitotic fidelity. He then moved to San Diego to work as a postdoctoral researcher at the Ludwig Institute for Cancer Research where he studied how mechanisms of cell division are impacted by development.
Research
CELL CYCLE DECISIONS IN DEVELOPMENT
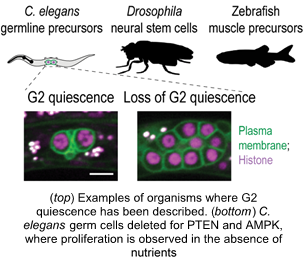
During development, one of the most important decisions that cells make is whether to continue proliferate or to enter a state of cell cycle pause known as cell cycle quiescence. Cells enter and exit quiescence in response to developmental cues or nutrient signaling. Failure of cells to quiesce results in tumorigenesis, whereas premature quiescence establishment can lead to developmental arrest.
Most prior work has focused on the G0 quiescent state that is established after cells exit mitosis. However, certain stem cell populations quiesce at the G2 stage of the cell cycle, right after the completion of DNA replication. But despite its emerging prevalence across a wide evolutionary spectrum, the mechanisms that allow for the establishment of G2 quiescence and how cells exit the G2 quiescence state to resume proliferation remain understudied.
Using a combination of cell biology, proteomics, genetic screens, and developmental analyses we are aiming to answer three key questions:
1. What mechanisms control entry into G2 quiescence?
2. How does external signaling regulate exit from G2 quiescence?
3. How do these signals interface with the cell cycle?
CONTROL OF MITOTIC FIDELITY
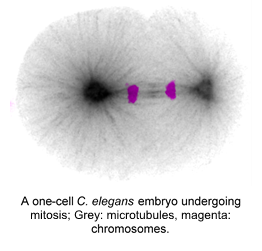
During mitosis, it is critical that duplicated chromosomes are equally partitioned between the two daughter cells. A mechanism known as the spindle assembly checkpoint ensures that cells spend enough time in mitosis until all chromosomes have attached to microtubules of the mitotic spindle. However, it is well known that the spindle assembly checkpoint does not play a significant role in regulating mitotic duration in embryonic cell cycles, indicating that there are other mechanisms at play.
While a minimal mitotic duration is important to prevent chromosome segregation errors, an increase in mitotic duration can, surprisingly, have detrimental consequences for embryonic viability. Indeed, extending mitotic duration can result in cell cycle arrest and embryonic lethality. Therefore, a big challenge of mitosis is balancing the need to ensuring accurate chromosome segregation while minimizing the time spent in a vulnerable cell cycle phase.
Using a combination of biochemistry and live-cell imaging we are working on understanding:
1. What molecular mechanisms regulate the timing of mitosis?
2. How do these mechanisms interface with the spindle assembly checkpoint?
3. What is the importance of mitotic timing control in development?
Recent publications:
For a complete list of publications, see: https://scholar.google.com/citations?user=18GSOZ8AAAAJ&hl=en
1. APC7 mediates ubiquitin signaling in constitutive heterochromatin in the developing mammalian brain
Ferguson C.J., Urso O., Bodrug T., Gassaway B.M., Watson E.R., Prabu J.R., Lara-Gonzalez P., Martinez-Chacin R.C., Wu D.Y., Brigatti K.W., Puffenberger E.G., Taylor C.M., Haas-Givler B., Jinks R.N., Strauss K.A., Desai A., Gabel H.W., Gygi S.P., Schulman B.A., Brown N.G., Bonni A.
Molecular Cell (2022), 82: 90-105
2. Spindle assembly checkpoint activation and silencing at kinetochores
Lara-Gonzalez P.*, Pines J.*, Desai A.*
Seminars in Cell & Developmental Biology (2021), 117: 86-98 (*corresponding authors) (review).
3. The N-terminal Tail of C. elegans CENP-A Interacts with KNL-2 and is Essential for Centromeric Chromatin Assembly
de Groot C., Houston J., Davis B., Gerson-Gurwitz A., Monen J., Lara-Gonzalez P., Oegema K., Shiau A.K., Desai A.
Molecular Biology of the Cell (2021), 32: 1193-1201.
4. A tripartite mechanism catalyses Mad2-Cdc20 assembly at unattached kinetochores.
Lara-Gonzalez P.*, Kim T., Oegema K., Corbett K., Desai A*.
Science (2021), 371, 64-67(*corresponding authors)
5. The PHLPP1 N-Terminal Extension is a Mitotic Cdk1 Substrate and Controls an Interactome Switch.
Kawashima A.T., Wong C., Lorden G., King C.C., Lara-Gonzalez P., Desai A., Gingras A.-C., Newton A.C.
Molecular and Cellular Biology (2021), 41, e00333-20.
6. BUB-1 targets PP2A:B56 to regulates chromosome congression during meiosis I in C. elegans oocytes
Bel Borja L., Soubigou F., Taylor S.J., Fraguas Bringas C., Budrewicz J., Lara-Gonzalez P., Sorensen Turpin C.G., Bembenek J.N., Cheerambathur D.K., Pelisch F.
eLife (2020), 9, e65307.
7. Rashomon at the kinetochore: function(s) of the Mad1-cyclin B1 complex.
Houston J., Lara-Gonzalez P., Desai A.
Journal of Cell Biology (2020), 219: e202006006 (review)
8. A non-canonical BRCT-phosphopeptide recognition mechanism underlies RhoA activation in cytokinesis.
Gomez-Cavazos J.S., Lee K.Y., Lara-Gonzalez P., Li Y., Desai A., Shiau, A.K., Oegema K.
Current Biology (2020), 30: 3101-315.
9. The G2-to-M transition is ensured by a dual mechanism that protects cyclin B from degradation by Cdc20-activated APC/C.
Lara-Gonzalez P.*, Moyle M.W., Budrewicz. J., Mendoza-Lopez J., Oegema K., Desai A*.
Developmental Cell (2019), 15: 313-325 (*corresponding authors).
10. Pharmacological convergence reveals a lipid pathway that regulates C. elegans lifespan.
Chen A.L., Lum K.M., Lara-Gonzalez P., Ogasawara D., Cognetta A.B. 3rd., To A., Parsons W.H., Simon G.M., Desai A., Petrascheck M., Bar-Peled L., Cravatt B.
Nature Chemical Biology (2019), 15: 453-462.
11. CFI-400945 is not a selective cellular PLK4 inhibitor.
Oegema K., Davis R.L., Lara-Gonzalez P., Desai A., Shiau A.K.
Proceeding of the Natural Academy of Sciences USA (2018), 115: E10808-E10809.
12.Employing the one-cell C. elegans embryo to study cell division processes.
Hattersley N.*, Lara-Gonzalez P.*, Cheerambathur D., Gomez-Cavazos J.S., Kim T., Prevo B., Khaliullin R., Lee K.Y., Ohta M., Green R., Oegema K., Desai A.
Methods in Cell Biology (2018), 144: 185-231 (*co-first authors).
13.Taming the Beast: Control of APC/CCdc20-Dependent Destruction.
Lara-Gonzalez P., Kim T., Desai A.
Cold Spring Harbor Symposium on Quantitative Biology (2017), 81. pii: 033712 (review).
14. Kinetochores accelerate or delay APC/C activation by directing Cdc20 to opposite fates.
Kim T.*, Lara-Gonzalez P.*, Prevo B., Meitinger F., Cheerambathur D., Oegema K., Desai A.
Genes & Development (2017), 31: 1089-1094 (*co-first authors).
Cover article: http://genesdev.cshlp.org/content/31/11.cover-expansion
15. A toolkit for GFP-mediated tissue-specific protein degradation in C. elegans.
Wang S., Tang N.H., Lara-Gonzalez P., Zhao Z., Prevo B., Cheerambathur D.K., Chisholm A.D., Desai A., Oegema K.
Development (2017), 144: 2694-2701.


