Depts of Anatomy & Neurobiology, Developmental & Cell Biology, and Center for Complex Biological Systems
University of California Irvine
2206 Biological Sciences III
Irvine, CA 92697
Tel: (949) 824-4616, 5745
Fax: (949) 824-1104
Email: alcalof@uci.edu
Systems Biology of Stem Cells and Human Developmental Disorders–The Calof lab uses genetics, molecular biology and systems biology approaches to address questions in development, regeneration, and tissue homeostasis. We also work on vertebrate animal model systems to try to understand the etiology of certain human genetic diseases that affect development and function, especially of the nervous system.
Among the questions we are addressing are:
How can we use molecular, cellular, and computational approaches together to gain insights into the behaviors of endogenous stem cells during development and regeneration of epithelia, particularly neural (sensory) epithelia? In particular, we are interested in understanding how interacting feedback loops mediated by different molecular signals interact to achieve the performance objectives of developing and regenerating epithelia. We move between wet-lab experimental approaches, to modeling and back again to experimental approaches, to test these ideas.
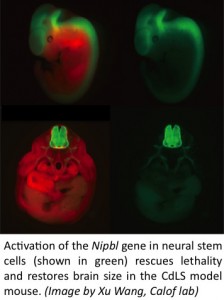 Can mouse and zebrafish models of Cornelia de Lange Syndrome provide insights into the many structural and physiological disorders, also common in non-syndromic birth defects, which are associated with this human genetic disease? Of particular interest are heart defects, limb reduction defects, and neural pathologies.
Can mouse and zebrafish models of Cornelia de Lange Syndrome provide insights into the many structural and physiological disorders, also common in non-syndromic birth defects, which are associated with this human genetic disease? Of particular interest are heart defects, limb reduction defects, and neural pathologies.
Are the developmental phenotypes observed in birth defects disorders (e.g. heart defects, autism) the result of the combinatorial actions of small changes in activities of many genes, rather than major changes in the activity of any one single gene? We use conditional/invertible alleles (FLEX alleles) of genes, in combination with unbiased transcriptome screens, to help us understand how small changes in gene expression that take place in particular locations and/or at particular developmental epochs, lead to widespread structural birth defects.
Recent papers of interest:
- Lander, A.D., Gokoffski, K.K., Wan, F., Nie, Q., and Calof, A.L. (2009) Cell Lineages and the logic of proliferative control. PLoS Biology 7(1): e1000015. doi:10.1371/journal.pbio.1000015. ** Reviewed in G.T. Reeves & S.E. Fraser (2009) “Biological Systems from an Engineer’s Point of View”, PLoS Biology Primer, PLoS Biol 7(1): e1000021.
- Kawauchi, S., Kim, J., Santos, R., Wu, H. H., Lander, A. D. and Calof, A. L. (2009). Foxg1 promotes olfactory neurogenesis by antagonizing Gdf11. Development 136, 1453-64.
- Beites, CL, Hollenbeck, PLW, Kim, J, Lovell-Badge, R, Lander, AD, and Calof, AL (2009) Follistatin modulates a BMP autoregulatory loop to control size and patterning of sensory domains in the developing tongue. Development, 136: 2187-2197.
- Kawauchi, S, Calof, AL**, Santos, R., Lopez-Burks, ME, Young, CM, Hoang, MP, Chua, A., Lao, T., Lechner, MS, Daniel, JA, Nussenzweig, A., Kitzes, L., Yokomori, K., Hallgrimsson, B. and Lander, A.D. (2009) Multiple organ system defects and transcriptional dysregulation in a mouse model of Cornelia de Lange Syndrome. PLoS Genetics 5(9): e1000650. Doi:10.1371/journal.pgen.1000650.
- Gokoffski, K.K., Kawauchi, S., H.-H. Wu, R. Santos, P.W.L. Hollenbeck, A.D. Lander and L. Calof (2010) “Feedback regulation of neurogenesis in the mammalian olfactory epithelium: New insights from genetics and systems biology.” In: Neurobiology of Olfaction, (A. Menini, Ed.) CRC Press, pp. 241-265. PMID:21882434.
- Gokoffski K, Wu H-H, Beites CL, Kim J, Kim E, Matzuk MM, Johnson JE, Lander AD & Calof AL (2011) Activin and GDF11 collaborate in feedback control of neuroepithelial stem cell proliferation and fate. Development, 138: pp. 4131-4142.
- Muto A, Calof AL, Lander AD, and Schilling, T.F. (2011) Multifactoral origins of heart and gut defects in nipbl-deficient zebrafish, a model of Cornelia de Lange Syndrome. PLoS Biology Oct;9(10):e1001181. Epub 2011 Oct 25. (comment: PLoS Biol. 2011 Oct;9(10):e1001180) PMCID: PMC3201921. Reviewed in J. Weaver (2011) “Genetic Origins of Birth Defects Revealed by New Animal Model”, PLoS Biology, (http://journals.plos.org/plosbiology/article?id=10.1371/journal.pbio.1001180).
- Santos R, Wu H-H, Hamilton J, Pinter R, Hindges R & Calof, AL (2012) Restoration of retinal development in Vsx2 deficient mice by reduction of Gdf11 activity. Adv. Exp. Med. Biol. 723: 671-677. PMID 22183392
- Lander AD, Kimble J, Clevers H, Fuchs E, Montarras D, Buckingham M, Calof AL, Trumpp A, and Oskarsson T (2012) What does the concept of the stem cell niche really mean today? BMC Biology, 10, 19: pp 1-15; doi: 10.1186/1741-7007-10-19.
- Remeseiro, A. Cuadrado, S. Kawauchi, A.L. Calof, A.D. Lander and A. Losada (2013) Reduction of Nipbl impairs cohesin loading locally and affects transcription but not cohesion-dependent functions in a mouse model of Cornelia de Lange Syndrome. Biochim Biophys Acta 1832, 2097-102. doi: 10.1016/j.bbadis.2013.07.020. PMCID: PMC3825806.
- Muto, A., S. Ikeda, M.E. Lopez-Burks, Y Kikuchi, L. Calof*, A.D. Lander and T.F. Schilling (2014) Nipbl and Mediator Cooperatively Regulate Gene Expression to Control Limb Development. PLoS Genetics, 10(9):e1004671. doi: 10.1371/journal.pgen.1004671. PMID 25255084. PMCID: PMC4177752.
- Chiang M, Hallman S, Cinquin A, de Mochel NR, Paz A, Kawauchi S, Calof AL, Cho KW, Fowlkes CC, Cinquin O. (2015) Analysis of in vivo single cell behavior by high throughput, human-in-the-loop segmentation of three-dimensional images. BMC Bioinformatics 16: https://doi.org/10.1186/s12859-015-0814-7 PMID: 26607933, PMCID: PMC4659165
- Kunche, S., H. Yan, L. Calof*, J.S. Lowengrub and A.D. Lander (2016) Feedback, lineages and self-organizing morphogenesis. PLoS Computational Biol, 12, e1004814. doi:10.1371/journal.pcbi.1004814. Cited in PLoS Collections, Editor’s Picks in Stem Cell Research (http://collections.plos.org/stem-cell-research).
- Kawauchi, S., R. Santos, A. Muto, M.E. Lopez-Burks, T.F. Schilling, A.D. Lander and L. Calof (2016) Using Mouse and Zebrafish Models to Understand the Etiology of Developmental Defects in Cornelia de Lange Syndrome. Am J Med Genet C Semin Med Genet. 172(2):138-45. doi: 10.1002/ajmg.c.31484. PMID: 27120001.
- Santos R., S. Kawauchi, R.E. Jacobs, M.E. Lopez-Burks, H. Choi, J. Wikenheiser, B. Hallgrimsson, H.A. Jamniczky, S.E. Fraser, A.D. Lander, and L. Calof* (2016) Conditional creation and rescue of Nipbl-deficiency in mice reveals multiple determinants of risk for congenital heart defects. PLOS Biology, 14, e2000197. doi: 10.1371/journal.pbio.2000197. PMID: 27606604. Reviewed in: PLoS Biology Primer by B. Gelb (2016)“The Hole and the Whole: Lessons from Manipulation of Nipbl Deficiency” 2016 PLoS Biology, doi: 10.1371/journal.pbio.2000494
- Newkirk, D., Y.-Y. Chen, R. Chien, W. Zeng, J. Biesinger, E. Flowers, S. Kawauchi, R. Santos, L. Calof, A.D. Lander, X. Xie, K. Yokomori (2017) The effect of Nipped-B-like (Nipbl) haploinsufficiency on genome-wide cohesin binding and target gene expression: modeling Cornelia de Lange Syndrome. Clinical Epigenetics, 2017 Aug 25; 9:89. doi: 10.1186/s13148-017-0391-x. eCollection 2017.
- Crestina L. Beites, Shimako Kawauchi, Rosaysela Santos, Kimberly K. Gokoffski, and Anne L. Calof (2017) Patterning, cell specification and feedback in the olfactory epithelium. In: Reference Module in Neuroscience and Biobehavioral Psychology, Elsevier, 2017, pp. 1-14; ISBN 978-0-12-809324-5, https://doi.org/10.1016/B978-0-12-809324-5.02621-3.
- Calof AL, Santos R, Groves L, Oliver C, and Lander AD. “Cornelia de Lange Syndrome: Insights into Neural Development from Clinical Studies and Animal Models.” In: Comprehensive Developmental Neurosciences Series, 2nd Edition, Neural Developmental Disorders Section (Anthony Winshaw-Boris, Editor), Elsevier Press. In preparation.
- Calof AL, Maclean A, Kopez-Burks M, Villagomez L, Bui B, Santos R, and Lander AD. Transcriptomic Approach to Identify Therapies and Therapeutic Targets for Cornelia de Lange Syndrome. In preparation.



