
Academic distinctions:
LEO Foundation award, Region Americas, 2019
American Association of Anatomists young investigator award, 2018
Pew Biomedical scholar, 2016
Edward Mallinckrodt, Jr. Foundation grantee, 2013
Dermatology Foundation grantee, 2013
Mechanisms of regeneration and stem cell control
Our laboratory studies how complex tissues and organs regenerate under normal conditions and in response to injury or disease. We aim to understand:
1. The nature of stem cell regulatory networks
Homeostasis of many organs largely depends on the activities of tissue-specific stem cells. In the past, a lot of research has been focused on understanding how individual stem cells respond to signals from their micro-environment and decide between remaining quiescent and becoming activated. However, it was unclear if and how thousands of stem cells can coordinate their activities with one another.
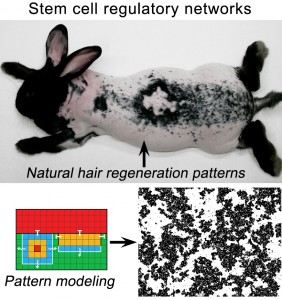 Recently, we were able to study collective behavior of adult stem cells using the model of hair regeneration. Each hair has a prominent cluster of stem cells. Since there are thousands of hairs on the surface of the skin and skin is flat, together all hair stem cells form a two-dimensional network of clusters. Within this network each stem cell cluster “listens” to competing activating and inhibitory signals and decides between remaining quiescent and becoming activated based on the combined signaling message that it receives. Because the decision making rules are similar for every stem cell cluster, scaling of this behavior across the entire network results in striking patterns of hair regeneration.
Recently, we were able to study collective behavior of adult stem cells using the model of hair regeneration. Each hair has a prominent cluster of stem cells. Since there are thousands of hairs on the surface of the skin and skin is flat, together all hair stem cells form a two-dimensional network of clusters. Within this network each stem cell cluster “listens” to competing activating and inhibitory signals and decides between remaining quiescent and becoming activated based on the combined signaling message that it receives. Because the decision making rules are similar for every stem cell cluster, scaling of this behavior across the entire network results in striking patterns of hair regeneration.
To this end, we developed a mathematical approach that enables predictive modeling of the hair regeneration patterns. Using predictive power of the model, we showed how key BMP and WNT signaling pathways from the stem cell micro-environment become reused to mediate long-range communication between neighboring hair stem cell clusters. Currently we are interested in the following questions:
A) In addition to WNT and BMP, what other key signaling pathways are co-opted to regulate large-scale regeneration of hair stem cells? We are using the predictive computational modeling coupled with in vivo validation experiments to identify new players in the hair stem cell signaling network.
B) Does large-scale coordination exist among stem cells in tissues other than skin? We are looking to identify the analogous two- and three-dimensional stem cell networks in other organs.
C) Can stem cell coordination be modulated to design more physiologically-relevant stem cell-based therapies?
2. Regenerative behavior in response to organ injury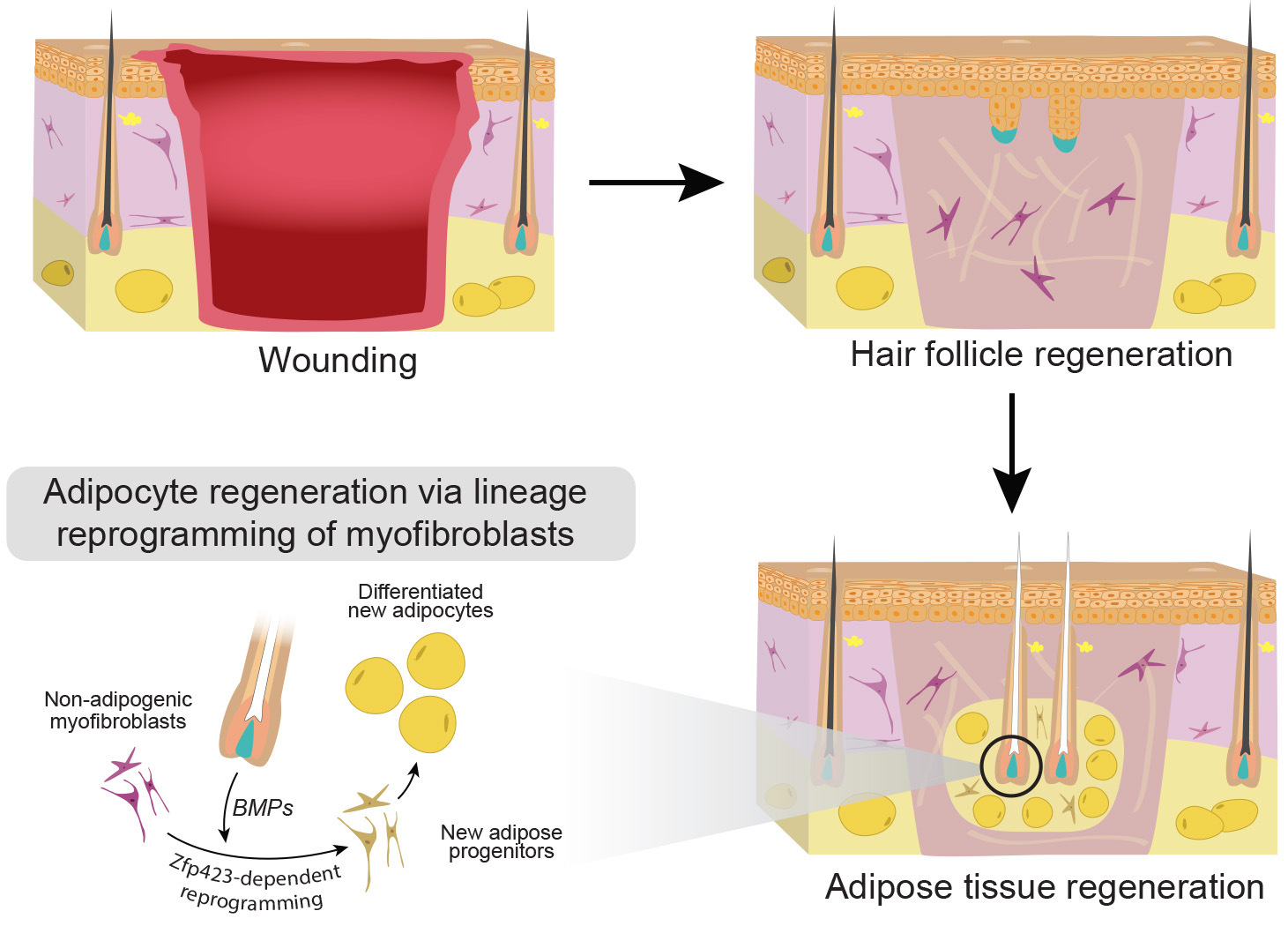 Our laboratory is also interested to understand the natural limits of adult cells’ plasticity in response to injury. Our ongoing work shows that the regenerative abilities of adult mammalian skin are far greater than previously thought. In the center of large skin wounds cells can acquire an embryonic-like state and develop new, fully functioning hair follicles. New adipose tissue regenerates around these hair follicles. Recently we showed that new adipocytes regenerate from myofibroblasts, scar-forming cells thought to be non-adipogenic. Myofibroblasts convert to adipocytes via the process of lineage reprogramming. Such reprogramming is triggered by hair-derived BMP signals, that activate adipocyte-specific transcription factors. Collectively, regenerative events can be so efficient that several months after wounding, scar tissue can hardly be distinguished from the normal skin. Using this regeneration model, we are studying:
Our laboratory is also interested to understand the natural limits of adult cells’ plasticity in response to injury. Our ongoing work shows that the regenerative abilities of adult mammalian skin are far greater than previously thought. In the center of large skin wounds cells can acquire an embryonic-like state and develop new, fully functioning hair follicles. New adipose tissue regenerates around these hair follicles. Recently we showed that new adipocytes regenerate from myofibroblasts, scar-forming cells thought to be non-adipogenic. Myofibroblasts convert to adipocytes via the process of lineage reprogramming. Such reprogramming is triggered by hair-derived BMP signals, that activate adipocyte-specific transcription factors. Collectively, regenerative events can be so efficient that several months after wounding, scar tissue can hardly be distinguished from the normal skin. Using this regeneration model, we are studying:
A) What mechanism allows lineage-restricted adult cells to expand their developmental plasticity in response to wounding?
B) How can embryonic-like regeneration be enhanced to achieve scarless healing of adult tissues?
Journal Covers
 |
 |
 |

|
Selected Publications See complete list here
- Fibroblasts: origins, definitions, and functions in health and disease
Plikus MV#, Wang X*, Sinha S*, Forte E*, Thompson SM*, Herzog EL#, Driskell RR#, Rosenthal N#, Biernaskie J#, Horsley V#
Cell 2021, 184(15): 3852-3872
- Genomic and anatomical comparisons of skin support independent adaptation to life in water by cetaceans and hippos
Springer MS*#, Guerrero-Juarez CF*, Huelsmann M, Collin M, Danil K, McGowen M, Oh JW, Ramos R, Hiller M#, Plikus MV#, Gatesy J#
Current Biology 2021, J.CUB.2021.02.057
UCI News: How did hippo, whale and dolphin skin adapt to live in water? UCI study reveals evolutionary clues News coverage by American Museum of Natural History, Nature World News, Earth.com
- Inference and analysis of cell-cell communication using CellChat
Jin S, Guerrero-Juarez CF, Zhang L, Chang I, Ramos R, Kuan C-H, Myung P, Plikus MV#, Nie Q#
Nature Communications 2021, 12: 1088
UCI News: UCI researchers eavesdrop on cellular conversations
LEO Foundation: Under the skin
- Single-cell analysis reveals fibroblast heterogeneity and myeloid-derived adipocyte progenitors in murine skin wounds
Guerrero-Juarez CF, Dedhia PH, Jin S, Ruiz-Vega R, Ma D, Liu Y, Yamaga K, Shestova O, Gay DL, Yang Z, Kessenbrock K, Nie Q, Pear WS, Cotsarelis G#, Plikus MV#
Nature Communications 2019, s41467-018-08247-x
UCI News: UCI-Led Study Reveals How Blood Cells Help Wounds Heal Scar-Free
News coverage by University of California
Highlight by National Science Foundation
- Pharmacological activation of REV-ERBs is lethal in cancer and oncogene-induced senescence
Sulli G, Rommel A, Wang X, Kolar MJ, Puca F, Saghatelian A, Plikus MV, Verma IM, Panda S
Nature 2018, Nature25170
- A multi-scale model for hair follicles reveals heterogeneous domains driving rapid spatiotemporal hair growth patterning
Wang Q*, Oh JW*, Lee HL, Dhar A, Peng T, Ramos R, Guerrero-Juarez CF, Wang X, Zhao R, Cao X, Le J, Fuentes MA, Jocoy SC, Rossi AR, Vu B, Pham K, Wang X, Mali NM, Park JM, Choi JH, Lee H, Legrand J, Kandyba E, Kim JC, Kim M, Foley J, Yu Z, Kobielak K, Andersen B, Khosrotehrani K, Nie Q#, Plikus MV#
eLife 2017, eLife.22772
eLife Insight: Tissue Regeneration: Regional differences
PEW Analysis: The Mystery of Hair Growth, Untangled
UCI News: UCI study sheds light on regulation of hair growth across the entire body
News coverage by University of California
Related press coverage in New Scientist, Medical News Today, Laboratory Equipment Magazine, Esquire, OC Register, Daily Mirror, Daily Mail, Birmingham Mail
- Regeneration of fat cells from myofibroblasts during wound healing
Plikus MV#, Guerrero-Juarez CF, Ito M, Li YR, Dedhia PH, Zheng Y, Shao M, Gay DL, Ramos R, Hsi TC, Oh JW, Wang X, Ramirez A, Konopelski SE, Elzein A, Wang A, Supapannachart RJ, Lee HL, Lim CH, Nace A, Guo A, Treffeisen E, Andl T, Ramirez RN, Murad R, Offermanns S, Metzger D, Chambon P, Widgerow AD, Tuan TL, Mortazavi A, Gupta RK, Hamilton BA, Millar SE, Seale P, Pear WS, Lazar MA, Cotsarelis G#
Science 2017, 355: 748-752
Perspective: Fibroblasts become fat to reduce scarring
Perspective: Repeal and replace: adipocyte regeneration in wound repair
UCI News: Study reveals natural process for scar-free wound healing
News coverage by University of California
Related press coverage in Popular Mechanics, Scientific American, Business Insider, The Times, The Guardian, Daily Mail, Yahoo! Beauty, OC Register, Skin Inc.
- Organ-level quorum sensing directs regeneration in hair stem cell populations
Chen CC, Wang L, Plikus MV, Jiang TX, Murray PJ, Ramos R, Guerrero-Juarez CF, Hughes MW, Lee OK, Shi S, Widelitz RB, Lander AD, Chuong CM
Cell 2015, 161: 277–290
- Dermal adipocytes protect against invasive Staphylococcus aureus skin infection
Zhang LJ, Guerrero-Juarez CF, Hata T, Bapat SP, Ramos R, Plikus MV, Gallo RL
Science 2015, 347: 67-71
UCI News: Skin fat is a newly recognized part of the body’s immune response
Orange County Register: Skin fat kills bacterial infections
- Local circadian clock gates cell cycle progression of transient amplifying cells during regenerative hair cycling
Plikus MV, Vollmers C, De la Cruz D, Chaix A, Ramos R, Panda S, Chuong CM
PNAS 2013, 10: E2106–E2115
Allure Magazine: Hair Fallout
Orange County Register: UCI In Focus: Researchers find timing is key for radiation treatment
- Fgf9 from dermal γδ T cells induces hair follicle neogenesis after wounding
Gay D, Kwon O, Zhang Z, Spata M, Plikus MV, Holler P, Ito M, Yang Z Treffeisen E, Kim C, Nace A, Zhang X, Baratono S, Wang F, Ornitz D, Millar SE, Cotsarelis G
Nature Medicine 2013, 19: 916-923
- Self-organizing and stochastic behaviors during the regeneration of hair stem cells
Plikus MV, Baker RE, Chen CC, Fare C, de la Cruz D, Andl T, Maini PK, Millar SE, Widelitz R, Chuong CM
Science 2011, 332: 586-589
- Cyclic dermal BMP signalling regulates stem cell activation during hair regeneration
Plikus MV, Mayer JA, de la Cruz D, Baker RE, Maini PK, Maxson R, Chuong CM
Nature 2008, 451: 340-344


